RADIAL TO PERIPHERAL (R2P) PORTFOLIO

PORTFOLIO OVERVIEW
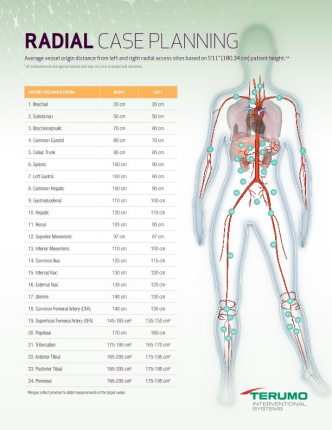
R2P® is the first and only portfolio of longer-length radial devices specifically designed for peripheral procedures, including above-the-knee PAD/CLI vascular interventions.
TERUMO continues to advance its product portfolio with R2P®, enabling you to utilize a comprehensive radial to peripheral approach to perform more peripheral procedures for more patients:

- Optimize entry-site management with Slender Technology
- Leverage the benefits associated with radial access for:
- Quicker ambulation, improved patient comfort, and satisfaction1-3
- Increased cath lab efficiencies and decreased overall costs per procedure4,5

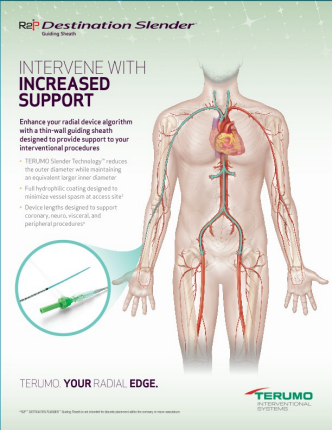
Slender Technology reduces the device outer diameter while maintaining larger inner diameter equivalent


- 5, 6, 7 Fr Sizes
- 10 cm and 16 cm lengths
Proprietary thin-wall technology and hydrophilic coating facilitate ease of insertion and removal during peripheral procedures


- 6 Fr Size
- 119 cm and 149 cm lengths
Fully hydrophilic coating allows for smooth transition within the radial artery and Slender TechnologyTM provides optimal performance during peripheral procedures


- 7 Fr Size
- 120 cm and 150 cm lengths
Guiding catheter with distal hydrophilic coating and Slender Technology for optimal performance during peripheral procedures

Provides the longest reach via the radial artery for peripheral procedures


- 0.035" Straight, Angled, 1.5 mm and 3 mm J Tip
- 350 cm, 400 cm, and 450 cm lengths
Includes multiple procedural options in shaft stiffness, tip shapes, and wire configurations for easier vessel navigation
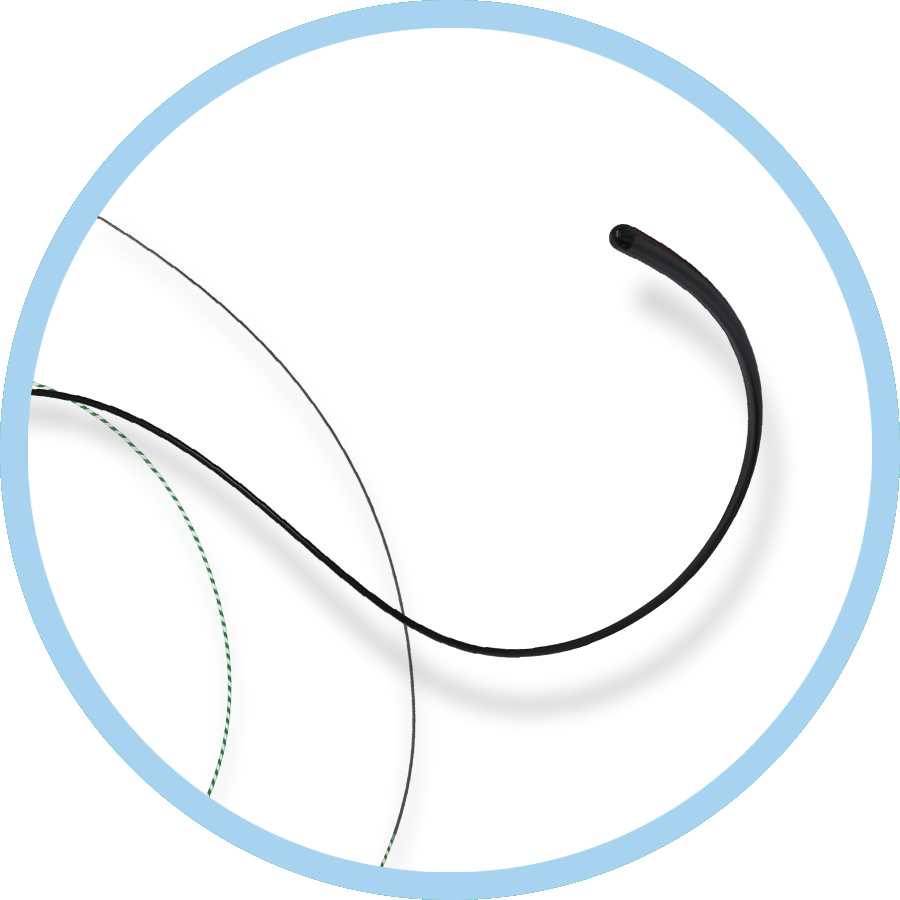

- 0.035” and 0.018”
- Angled and 1.5 mm J Tip
- 350 cm, 400 cm, 450cm and 500 cm lengths
Featuring the most comprehensive length offering of radial to peripheral wire (500cm) available.


- 4 Fr Size
- 150 cm length
Offers excellent pushability and torque control with multiple tip shapes for selectivity and access to the peripheral vasculature


- 4.5 Fr Size, Straight, Angled
- 200 cm length
- NAVICROSS also available in 135 cm and 150 cm
Optimal Torque Control - double braided stainless steel construction provides optimal torque control avoiding overshooting and minimizing delayed tip response

Rapid Exchange technology enables greater efficiency during peripheral procedures via the radial artery


- 6 Fr sheath compatibility
- 3-8 mm diameter x 20-200 mm length
- 200 cm shaft length
The longest 0.035" radial to peripheral capability and Rapid Exchange (RX) technology are designed to mitigate excessive device management and use of contrast media during a peripheral procedure


- 5 Fr sheath compatibility
- 2-6 mm diameter x 40-200 mm length
- 200 cm shaft length
The longest 0.018” Rapid Exchange (RX) PTA Balloon designed for use in radial to peripheral procedures
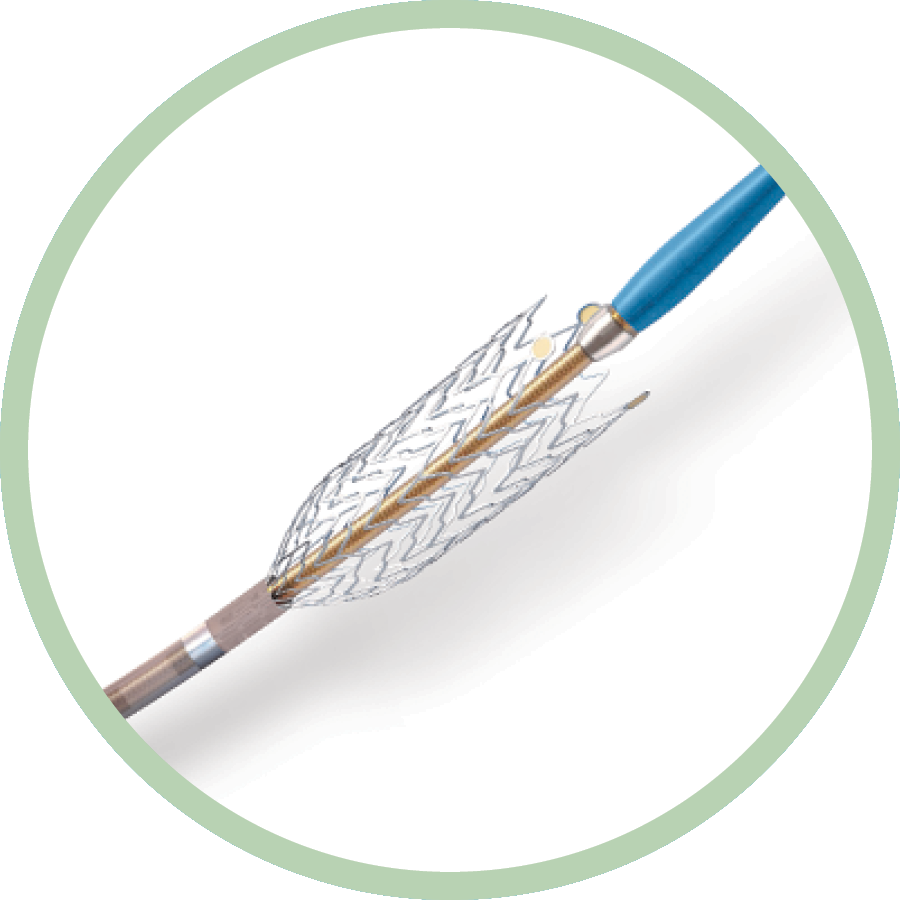

- 6 Fr sheath compatibility
- 6-8 mm diameter x 40-150 mm length
- 200 cm shaft length
The longest stent platform specifically designed for above-the-knee peripheral artery disease interventions via radial access with Rapid Exchange (RX) technology
Indications
The R2P®MISAGO® RX Self-expanding Peripheral Stent is indicated to improve luminal diameter in symptomatic patients with de novo or restenotic native lesions or occlusions of the Superficial Femoral Artery (SFA) and/or proximal popliteal artery with reference vessel diameters ranging from 4 mm to 7 mm and lesion length up to 150 mm.
Important Safety Information
Do not use this device in patients who exhibit angiographic evidence of severe thrombus in the target vessel or lesion site before/after undergoing Percutaneous Transluminal Angioplasty (PTA) procedure, patients with contraindication to antiplatelet and/or anticoagulation therapy, patients who are judged to have a lesion that prevents proper placement or deployment of the stent, a lesion that is within an aneurysm or an aneurysm with a proximal or distal segment to the lesion, or a lesion through which a guide wire cannot pass. This device should only be used by a physician who is familiar with, and well trained in, Percutaneous Transluminal Angioplasty (PTA) techniques, stent implantation, and transradial access.
RX ONLY. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.

Enables precise pressure to the artery following radial procedures

- Regular 24 cm and Long 29 cm
The #1 preferred radial hemostasis device on the market provides a more precise way of applying pressure to the radial artery
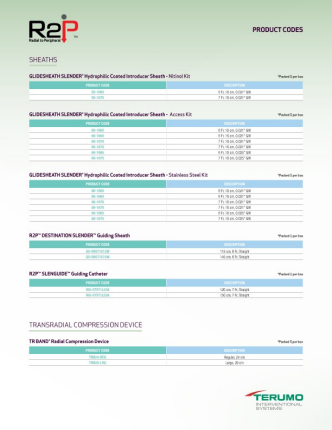
R2P® PORTFOLIO PRODUCT CODES
SHEATHS
GLIDESHEATH SLENDER® Hydrophilic Coated Introducer Sheath Nitinol Kit (5 per box)
| Product Code | Description |
| 50-1060 | 6 Fr, 10 cm, 0.021" GW |
| 50-1070 | 7 Fr, 10 cm, 0.021" GW |
GLIDESHEATH SLENDER® Hydrophilic Coated Introducer Sheath Access Kit (5 per box)
| Product Code | Description |
| 60-1060 | 6 Fr, 10 cm, 0.021" GW |
| 60-1660 | 6 Fr, 16 cm, 0.021" GW |
| 60-1070 | 7 Fr, 10 cm, 0.021" GW |
| 60-1670 | 7 Fr, 16 cm, 0.021" GW |
| 60-1065 | 6 Fr, 10 cm, 0.025" GW |
| 60-1075 | 7 Fr, 10 cm, 0.025" GW |
GLIDESHEATH SLENDER® Hydrophilic Coated Introducer Sheath Stainless Steel Kit (5 per box)
| Product Code | Description |
| 80-1060 | 6 Fr, 10 cm, 0.021" GW |
| 80-1660 | 6 Fr, 16 cm, 0.021" GW |
| 80-1070 | 7 Fr, 10 cm, 0.021" GW |
| 80-1670 | 7 Fr, 16 cm, 0.021" GW |
| 80-1065 | 6 Fr, 10 cm, 0.025" GW |
| 80-1075 | 7 Fr, 10 cm, 0.025" GW |
R2P® DESTINATION SLENDER® Guiding Sheath (1 per box)
| Product Code | Description |
| GS-R6ST1C12W | 119 cm, 6 Fr, Straight |
| GS-R6ST1C15W | 149 cm, 6 Fr, Straight |
R2P® SLENGUIDE® Guiding Catheter (1 per box)
| Product Code | Description |
| WG-S7ST1L23A | 120 cm, 7 Fr, Straight |
| WG-S7ST1L53A | 150 cm, 7 Fr, Straight |
GUIDEWIRES
R2P® Glidewire Advantage®
| Product Code | Description |
| GA3535 | 0.035", 350 cm, Angle |
| GA3540 | 0.035", 400 cm, Angle |
| GA3539 | 0.035", 400 cm, 1.5 mm J-tip |
| GR3535 | 0.035", 450 cm, Angle |
| GA3544 | 0.035", 450 cm, 1.5 mm J-tip |
| GA1840 | 0.018", 400 cm, 35° Angle |
| GA1845 | 0.018", 450 cm, 35° Angle |
| GA1850 | 0.018", 500 cm, 35° Angle |
GLIDEWIRE® Hydrophilic Coated Guidewire (5 per box)
| Product Code | Description |
| GR3536 | Standard, 0.035", 350 cm, Straight |
| GR3541 | Standard, 0.035", 400 cm, Straight |
| GR3546 | Standard, 0.035", 450 cm, Straight |
| GR3535 | Standard, 0.035", 350 cm, 45° Angle |
| GR3540 | Standard, 0.035", 400 cm, 45° Angle |
| GR3543 | Standard, 0.035", 450 cm, 45° Angle |
| GR3533 | Standard, 0.035", 350 cm, 1.5 mm J Tip |
| GR3539 | Standard, 0.035", 400 cm, 1.5 mm J Tip |
| GR3544 | Standard, 0.035", 450 cm, 1.5 mm J Tip |
| GR3534 | Standard, 0.035", 350 cm, 3 mm J Tip |
| GR3538 | Standard, 0.035", 400 cm, 3 mm J Tip |
| GR3545 | Standard, 0.035", 450 cm, 3 mm J Tip |
| GS3536 | Stiff, 0.035", 350 cm, Straight |
| GS3541 | Stiff, 0.035", 400 cm, Straight |
| GS3546 | Stiff, 0.035", 450 cm, Straight |
| GS3535 | Stiff, 0.035", 350 cm, 45° Angle |
| GS3540 | Stiff, 0.035", 400 cm, 45° Angle |
| GS3543 | Stiff, 0.035", 450 cm, 45° Angle |
| GS3533 | Stiff, 0.035", 350 cm, 1.5 mm J Tip |
| GS3539 | Stiff, 0.035", 400 cm, 1.5 mm J Tip |
| GS3544 | Stiff, 0.035", 450 cm, 1.5 mm J Tip |
CATHETERS
GLIDECATH® Hydrophilic Coated Catheter (5 per box)
| Product Code | Description |
| CG430 | 150 cm, 4 Fr, PV Multicurve |
| CG431 | 150 cm, 4 Fr, Straight |
| CG432 | 150 cm, 4 Fr, Angle |
| CG433 | 150 cm, 4 Fr, Pigtail |
| CG434 | 150 cm, 4 Fr, Cobra 2 |
| CG435 | 150 cm, 4 Fr, JR 4.0 |
| CG436 | 150 cm, 4 Fr, IMA |
R2P® NAVICROSS® Support Catheter (1 per box)
| Product Code | Description |
| SC-R35STR200 | Straight Tip, 0.035" x 200 cm |
| SC-R35ANG200 | Angled Tip, 0.035" x 200 cm |
NAVICROSS® Support Catheter (1 per box)
| Product Code | Description |
| NC35130 | Straight Tip, 0.035" x 135 cm |
| NC35131 | Angle Tip, 0.035" x 135 cm |
| NC35150 | Straight Tip, 0.035" x 150 cm |
| NC35151 | Angle Tip, 0.035" x 150 cm |
PTA BALLOON
R2P® METACROSS® RX PTA Balloon Dilatation Catheter (1 per box)
| Product Code | Description |
| BD-P30020ER | 200 cm, 6 Fr, 3 mm x 20 mm |
| BD-P30040ER | 200 cm, 6 Fr, 3 mm x 40 mm |
| BD-P30060ER | 200 cm, 6 Fr, 3 mm x 60 mm |
| BD-P30080ER | 200 cm, 6 Fr, 3 mm x 80 mm |
| BD-P30100ER | 200 cm, 6 Fr, 3 mm x 100 mm |
| BD-P30120ER | 200 cm, 6 Fr, 3 mm x 120 mm |
| BD-P30150ER | 200 cm, 6 Fr, 3 mm x 150 mm |
| BD-P30200ER | 200 cm, 6 Fr, 3 mm x 200 mm |
| BD-P40020ER | 200 cm, 6 Fr, 4 mm x 20 mm |
| BD-P40040ER | 200 cm, 6 Fr, 4 mm x 40 mm |
| BD-P40060ER | 200 cm, 6 Fr, 4 mm x 60 mm |
| BD-P40080ER | 200 cm, 6 Fr, 4 mm x 80 mm |
| BD-P40100ER | 200 cm, 6 Fr, 4 mm x 100 mm |
| BD-P40120ER | 200 cm, 6 Fr, 4 mm x 120 mm |
| BD-P40150ER | 200 cm, 6 Fr, 4 mm x 150 mm |
| BD-P40200ER | 200 cm, 6 Fr, 4 mm x 200 mm |
| BD-P50020ER | 200 cm, 6 Fr, 5 mm x 20 mm |
| BD-P50040ER | 200 cm, 6 Fr, 5 mm x 40 mm |
| BD-P50060ER | 200 cm, 6 Fr, 5 mm x 60 mm |
| BD-P50080ER | 200 cm, 6 Fr, 5 mm x 80 mm |
| BD-P50100ER | 200 cm, 6 Fr, 5 mm x 100 mm |
| BD-P50120ER | 200 cm, 6 Fr, 5 mm x 120 mm |
| BD-P50150ER | 200 cm, 6 Fr, 5 mm x 150 mm |
| BD-P50200ER | 200 cm, 6 Fr, 5 mm x 200 mm |
| BD-P60020ER | 200 cm, 6 Fr, 6 mm x 20 mm |
| BD-P60040ER | 200 cm, 6 Fr, 6 mm x 40 mm |
| BD-P60060ER | 200 cm, 6 Fr, 6 mm x 60 mm |
| BD-P60080ER | 200 cm, 6 Fr, 6 mm x 80 mm |
| BD-P60100ER | 200 cm, 6 Fr, 6 mm x 100 mm |
| BD-P60120ER | 200 cm, 6 Fr, 6 mm x 120 mm |
| BD-P60150ER | 200 cm, 6 Fr, 6 mm x 150 mm |
| BD-P60200ER | 200 cm, 6 Fr, 6 mm x 200 mm |
| BD-P70020ER | 200 cm, 6 Fr, 7 mm x 20 mm |
| BD-P70040ER | 200 cm, 6 Fr, 7 mm x 40 mm |
| BD-P70060ER | 200 cm, 6 Fr, 7 mm x 60 mm |
| BD-P70080ER | 200 cm, 6 Fr, 7 mm x 80 mm |
| BD-P70100ER | 200 cm, 6 Fr, 7 mm x 100 mm |
| BD-P70120ER | 200 cm, 6 Fr, 7 mm x 120 mm |
| BD-P70150ER | 200 cm, 6 Fr, 7 mm x 150 mm |
| BD-P70200ER | 200 cm, 6 Fr, 7 mm x 200 mm |
| BD-P80020ER | 200 cm, 6 Fr, 8 mm x 20 mm |
| BD-P80040ER | 200 cm, 6 Fr, 8 mm x 40 mm |
| BD-P80060ER | 200 cm, 6 Fr, 8 mm x 60 mm |
| BD-P80080ER | 200 cm, 6 Fr, 8 mm x 80 mm |
R2P® CROSSTELLA® RX PTA Balloon Dilatation Catheter (1 per box)
| Product Code | Description |
| BD-Q20040ER | 200 cm, 4 Fr, 2 mm x 40 mm |
| BD-Q20100ER | 200 cm, 4 Fr, 2 mm x 100 mm |
| BD-Q20150ER | 200 cm, 4 Fr, 2 mm x 150 mm |
| BD-Q20200ER | 200 cm, 4 Fr, 2 mm x 200 mm |
| BD-Q30040ER | 200 cm, 4 Fr, 3 mm x 40 mm |
| BD-Q30100ER | 200 cm, 4 Fr, 3 mm x 100 mm |
| BD-Q30150ER | 200 cm, 4 Fr, 3 mm x 150 mm |
| BD-Q30200ER | 200 cm, 4 Fr, 3 mm x 200 mm |
| BD-Q40040ER | 200 cm, 4 Fr, 4 mm x 40 mm |
| BD-Q40100ER | 200 cm, 4 Fr, 4 mm x 100 mm |
| BD-Q40150ER | 200 cm, 4 Fr, 4 mm x 150 mm |
| BD-Q40200ER | 200 cm, 4 Fr, 4 mm x 200 mm |
| BD-Q50040ER | 200 cm, 4 Fr, 5 mm x 40 mm |
| BD-Q50100ER | 200 cm, 4 Fr, 5 mm x 100 mm |
| BD-Q50150ER | 200 cm, 5 Fr, 5 mm x 150 mm |
| BD-Q50200ER | 200 cm, 5 Fr, 5 mm x 200 mm |
| BD-Q60040ER | 200 cm, 4 Fr, 6 mm x 40 mm |
| BD-Q60100ER | 200 cm, 5 Fr, 6 mm x 100 mm |
| BD-Q60150ER | 200 cm, 5 Fr, 6 mm x 150 mm |
| BD-Q60200ER | 200 cm, 5 Fr, 6 mm x 200 mm |
STENT
R2P® MISAGO® RX Self-expanding Peripheral Stent (1 per box)
| Product Code | Description |
| SXR06040R | 200 cm, 6 Fr, 6 mm x 40 mm |
| SXR06060R | 200 cm, 6 Fr, 6 mm x 60 mm |
| SXR06080R | 200 cm, 6 Fr, 6 mm x 80 mm |
| SXR06100R | 200 cm, 6 Fr, 6 mm x 100 mm |
| SXR06120R | 200 cm, 6 Fr, 6 mm x 120 mm |
| SXR06150R | 200 cm, 6 Fr, 6 mm x 150 mm |
| SXR07040R | 200 cm, 6 Fr, 7 mm x 40 mm |
| SXR07060R | 200 cm, 6 Fr, 7 mm x 60 mm |
| SXR07080R | 200 cm, 6 Fr, 7 mm x 80 mm |
| SXR07100R | 200 cm, 6 Fr, 7 mm x 100 mm |
| SXR07120R | 200 cm, 6 Fr, 7 mm x 120 mm |
| SXR07150R | 200 cm, 6 Fr, 7 mm x 150 mm |
| SXR08040R | 200 cm, 6 Fr, 8 mm x 40 mm |
| SXR08060R | 200 cm, 6 Fr, 8 mm x 60 mm |
| SXR08080R | 200 cm, 6 Fr, 8 mm x 80 mm |
| SXR08100R | 200 cm, 6 Fr, 8 mm x 100 mm |
TRANSRADIAL COMPRESSION DEVICE
TR BAND® Radial Compression Device (5 per box)
| Product Code | Description |
| TRB24-REG | Regular, 24 cm |
| TRB29-LRG | Large, 29 cm |
DOCUMENTS
RX ONLY. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.
Indications
The R2P® MISAGO® RX Self-expanding Peripheral Stent is indicated to improve luminal diameter in symptomatic patients with de novo or restenotic native lesions or occlusions of the Superficial Femoral Artery (SFA) and/or proximal popliteal artery with reference vessel diameters ranging from 4 mm to 7 mm and lesion length up to 150 mm.
Important Safety Information
Do not use this device in patients who exhibit angiographic evidence of severe thrombus in the target vessel or lesion site before/after undergoing Percutaneous Transluminal Angioplasty (PTA) procedure, patients with contraindication to antiplatelet and/or anticoagulation therapy, patients who are judged to have a lesion that prevents proper placement or deployment of the stent, a lesion that is within an aneurysm or an aneurysm with a proximal or distal segment to the lesion, or a lesion through which a guide wire cannot pass. This device should only be used by a physician who is familiar with, and well trained in, Percutaneous Transluminal Angioplasty (PTA) techniques, stent implantation, and transradial access.
REFERENCES
- Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Catheter Cardiovasc Interv. 2014;83(2):228-236.
- Cooper CJ, El-Shiekh RA, Cohen DJ, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J. 1999;138(3):430-436.
- Jolly SS, Yusuf S, Cairns J, et al; RIVAL Trial Group. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377(9775):1409-1420.
- Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157(1):132-140.
- Caputo RP, Tremmel JA, Rao S, et al. Transradial arterial access for coronary and peripheral procedures: executive summary by the Transradial Committee of the SCAI. Catheter Cardiovasc Interv. 2011;78(6):823-839.
- Data on file.