PROCEDURE OVERVIEW
Complex coronary artery disease is becoming more common, and can lead to challenges in treatment. Factors that contribute to categorizing a lesion as complex include severe calcification, location, and extreme tortuosity.
Given the challenges in treating coronary artery disease, it is especially important for physicians to be confident in choosing the correct tools.
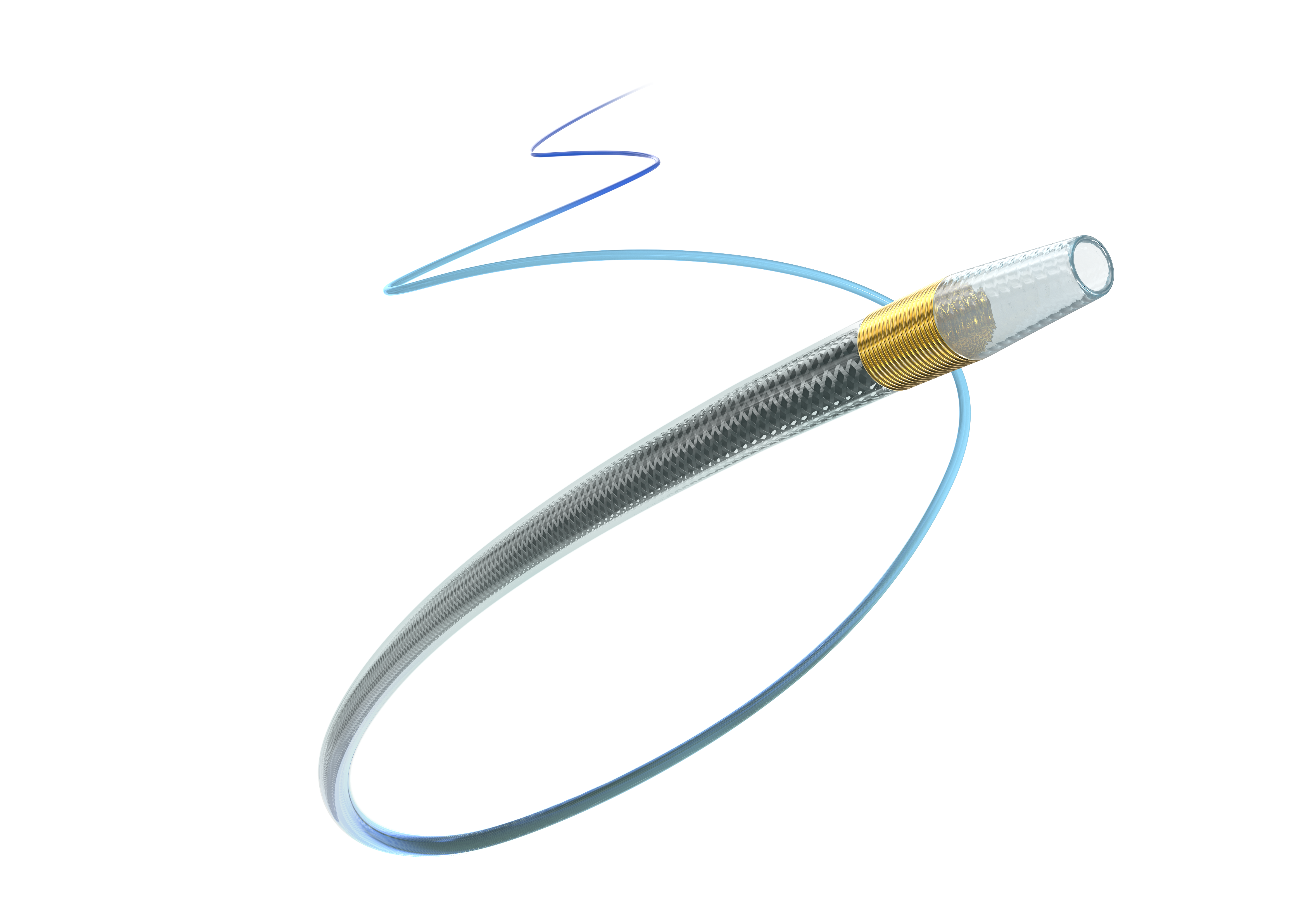
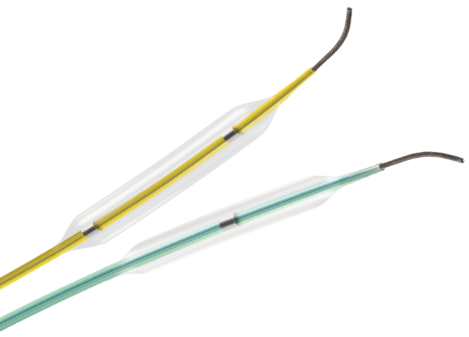
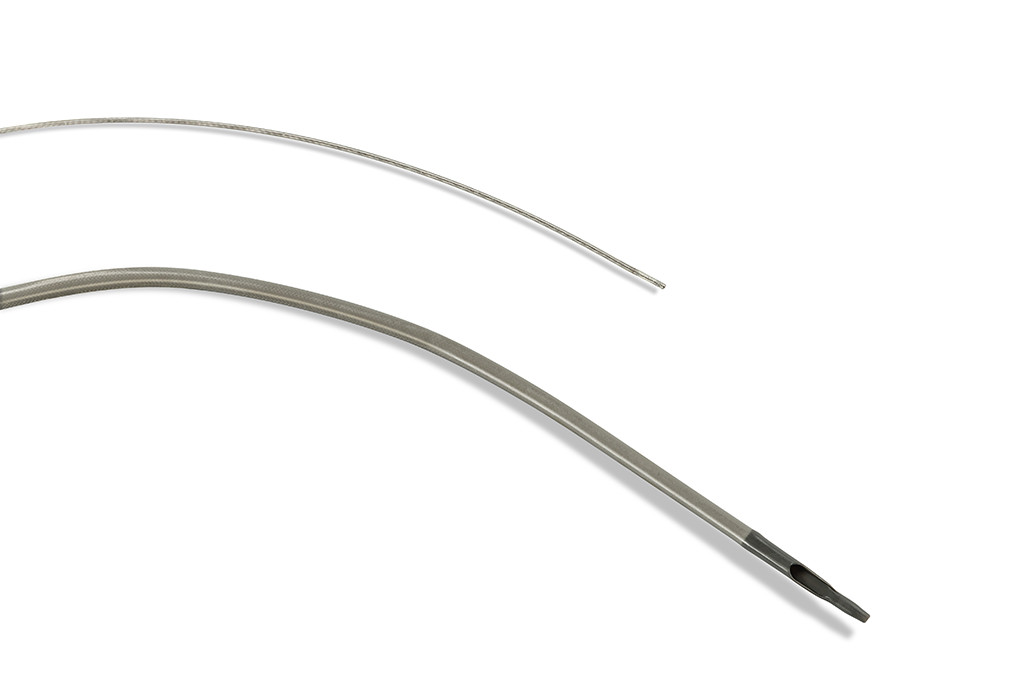
Our coronary procedural devices provide reliable access and closure, navigate through tortuous anatomy, and cross and treat complex lesions to improve patient outcomes.

PCI in 6 is brought to you by Terumo Interventional Systems focusing on the ability to discharge patients within 6 hours post PCI procedures.
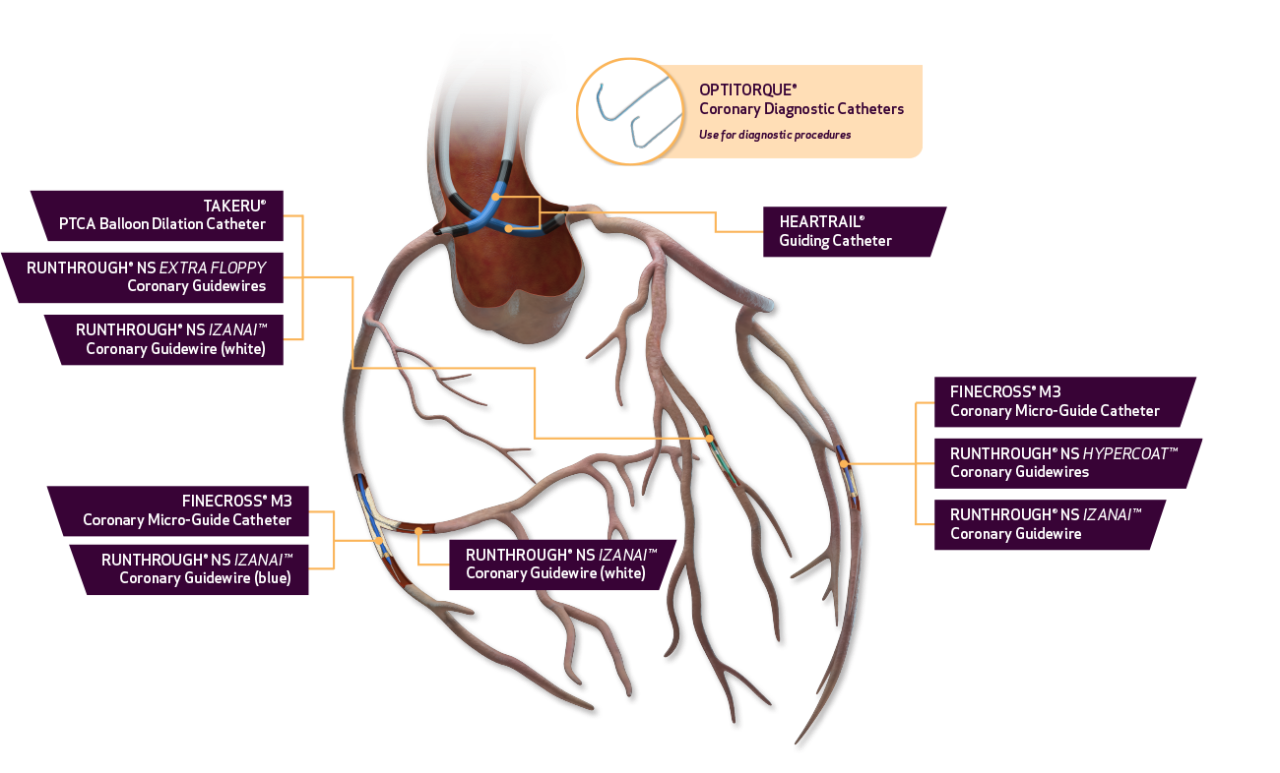
CORONARY PRODUCT PORTFOLIO
Terumo has solutions for all your needs from Access to Closure.
REFERENCES
RX ONLY. This advertisement is directed to physicians only, and not to consumers. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.
Indications:
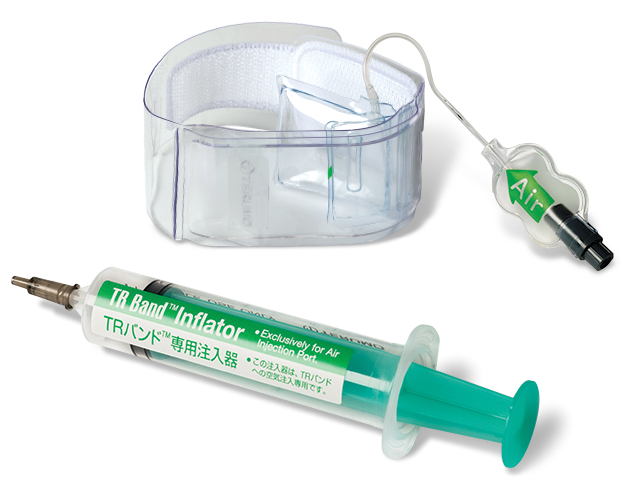
The Angio-Seal Vascular Closure Device is indicated for use in closing and reducing time to hemostasis of the femoral arterial puncture site in patients who have undergone diagnostic angiography procedures or interventional procedures using an 8 French or smaller procedural sheath for the 8 F Angio-Seal device and a 6 French or smaller procedural sheath for the 6 F Angio-Seal device. Angio-Seal is also indicated for use to allow patients who have undergone diagnostic angiography to safely ambulate as soon as possible after sheath removal and device placement, as well as to allow patients who have undergone an interventional procedure to safely ambulate after sheath removal and device placement.
Important Safety Information:
Possible adverse events for vascular closure devices include, but are not limited to: bleeding or hematoma, AV fistula or pseudoaneurysm, infection, allergic reaction, foreign body reaction, inflammation or edema. This device should only be used by physicians with training qualifying them to perform arterial access and closure for endovascular procedures through the common femoral artery and have participated in a Terumo Medical Corporation Angio-Seal physician instruction program.
Exception (applicable to US and China only): This device should only be used by a licensed physician (or other health care professional authorized by or under the direction of such physician) possessing adequate instruction in the use of the device, e.g., participation in an Angio-Seal physician instruction program or equivalent.