AZUR™ CX Peripheral Coil System
ADVANCED SOLUTIONS FOR COIL EMBOLIZATION IN GI BLEEDS AND BEYOND
OPTIMIZE OUTCOMES IN MORE PROCEDURES
2mm and 3mm sizes are available for small vessel embolization

USE WITH CONFIDENCE
Designed to be flexible and provides cross-sectional coverage
REDUCE COMPLICATIONS
AZUR CX has a solid core upon hydrogel expansion which, unlike thrombus, will not be absorbed by the body3
Hydrogel is unaffected by thrombolytic processes3
INCREASE EFFICIENCY
Use directly out of package: Does not require sterile warm saline or steam before insertion for hydration preparation3
COIL EMBOLIZATION GI BLEED SOLUTION WITH AZUR CX AND HYDROGEL EMBOLICS
Available in 2 mm and 3 mm sizes, the AZUR™ CX Peripheral Coil System delivers flexible, controlled coil embolization for GI bleeds and other small vessel procedures. These soft, hydrogel-coated embolics are designed for precision, durability, and versatility in a wide range of clinical indications.

CONTROLLED MECHANICAL OCCLUSION, LESS RELIANCE ON THROMBUS FORMATION1
The AZUR CX coil system achieves mechanical occlusion using a soft, flexible coil that allows for up to 30 minutes of repositioning (20 minutes for CX 0.035")*. This extended working time enables controlled delivery, particularly important in challenging embolization cases like gastrointestinal bleeds.

HYDROGEL EXPANSION FOR DENSE OCCLUSION
Solid Core Coil Embolics that Expand and Stay in Place
Designed to fill vessel lumens effectively, AZUR CX coils form a dense embolic mass as their hydrogel coating expands into vessel gaps. These embolics are not absorbed by the body, offering lasting occlusion in high-risk bleeding areas.1,2

EASY DEPLOYMENT WITH AZUR DETACHMENT SYSTEM
Enhanced Placement for Embolics in Complex Vessels
The AZUR Detachment System allows for precise placement, even in tortuous anatomy. The soft and flexible AZUR coil design minimizes catheter manipulation with a unique coil design3 and offers intuitive feedback for clinicians.
- Cross-coverage and anchoring features
- Reduced need for repositioning
- Consistent delivery of embolics in both small and large vessels

EXPAND YOUR COIL EMBOLIZATION OPTIONS WITH AZUR CX
From GI Bleeds to Peripheral Aneurysms, One System Covers More
2 mm and 3 mm coil sizes enable embolization in smaller vessels, increasing your ability to treat:
SMALL VESSEL COIL EMBOLIZATION:
- Gastrointestinal bleeds (GI bleed coil embolization)
- Pre-Y90 radioembolization
- Prostate artery embolization
- Other pelvic vasculature treatments
LARGE VESSEL EMBOLIZATION:
- Internal iliac artery procedures
- Pelvic congestion syndrome
- Varicocele embolization
- Peripheral aneurysms and AVMs
TREAT MORE PATIENTS WITH THE POTENTIAL TO USE FEWER COILS3

COMPLETE YOUR COIL EMBOLIZATION TOOLKIT
Explore the full line of embolics products from Terumo**, engineered for controlled, lasting vessel occlusion in both routine and complex interventions. Whether treating a GI bleed or embolizing a pelvic artery, AZUR CX gives you the precision, versatility, and confidence to take control.
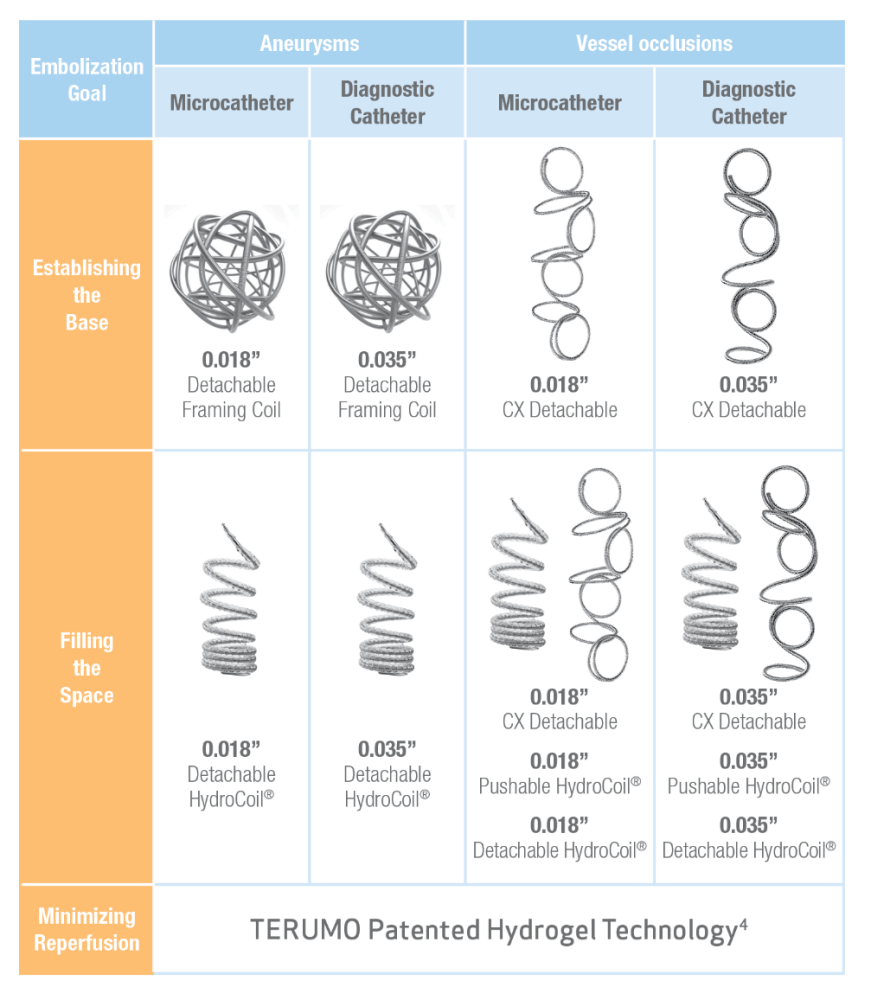
**For every vessel occlusion or aneurysm where a microcatheter or standard diagnostic catheter is required or preferred
IN-SERVICE VIDEO
PRODUCT CODES
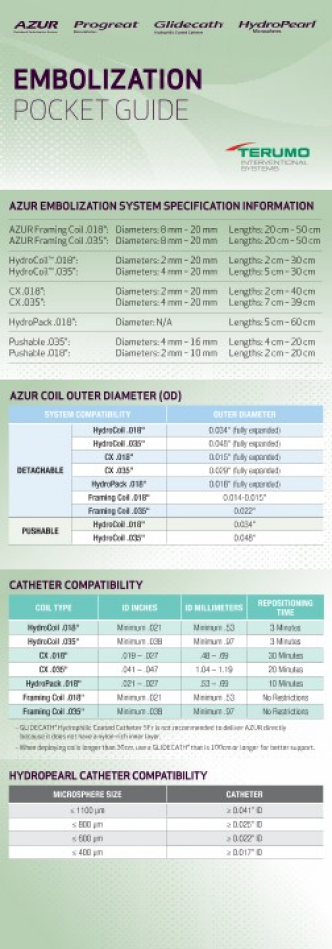
AZUR® CX Detachable 0.018” System / Pack of 1 - Product SKUs
| PRODUCT CODE | LOOP DIAMETER (mm) |
LENGTH† (cm) |
| 45-780202 | 2 | 2 |
| 45-780204 | 2 | 4 |
| 45-780304 | 3 | 4 |
| 45-780308 | 3 | 8 |
| 45-780413 | 4 | 13 |
| 45-780516 | 5 | 16 |
| 45-780620 | 6 | 20 |
| 45-780724 | 7 | 24 |
| 45-780828 | 8 | 28 |
| 45-780928 | 9 | 28 |
| 45-781032 | 10 | 32 |
| 45-781238 | 12 | 38 |
| 45-781434 | 14 | 34 |
| 45-781639 | 16 | 39 |
| 45-781836 | 18 | 36 |
| 45-782040 | 20 | 40 |
CATHETER ID REQUIREMENTS
| COIL TYPE | INCHES (min-max) |
MILLIMETERS (min-max) |
REPOSITIONING TIME |
| AZUR® CX 0.018" | .019 - .027 | .48 - .69 | 30 minutes |
| AZUR® CX 0.035" | .041 - .047 | 1.04 - 1.19 | 20 minutes |
DOCUMENTS
AZUR® CX Detachable 0.035” System / Pack of 1 - Product SKUs
| PRODUCT CODE | LOOP DIAMETER (mm) |
LENGTH† (cm) |
| 45-750407 | 4 | 7 |
| 45-750511 | 5 | 11 |
| 45-750609 | 6 | 9 |
| 45-750617 | 6 | 17 |
| 45-750812 | 8 | 12 |
| 45-750824 | 8 | 24 |
| 45-751019 | 10 | 19 |
| 45-751324 | 13 | 24 |
| 45-751632 | 16 | 32 |
| 45-752039 | 20 | 39 |
Detachable Controller for use with Detachable Systems / Pack of 5
| PRODUCT CODE | PRODUCT DESCRIPTION |
| 45-4001 | AZUR® Detachment Controller |
FREQUENTLY ASKED QUESTIONS
Q: What is coil embolization for a GI bleed?
A: Coil embolization for a GI bleed is a minimally invasive procedure that uses small metal coils, such as the AZUR™ CX or AZUR™ coil, to block bleeding blood vessels in the gastrointestinal tract. These coils act as embolics, forming a mechanical occlusion to stop bleeding and reduce reliance on natural clot formation.
Q: What are AZUR CX coils used for in GI bleed management?
A: AZUR™ CX coils are used for controlled coil embolization in GI bleeds and other vascular conditions. With hydrogel-coated embolics, the AZUR CX system expands to create a dense, lasting occlusion, making it ideal for small vessel embolization in the GI tract.
Q: How do AZUR coils work as embolics?
A: AZUR coils feature a hydrogel coating that expands after placement, filling gaps within the vessel for dense occlusion. These embolics are designed to remain in the body without being absorbed, providing reliable vessel closure during procedures like GI bleed embolization.
Q: Are AZUR CX coils effective for small vessel embolization?
A: Yes, AZUR CX coils, available in 2 mm and 3 mm sizes, are specifically designed for small vessel embolization, including use in GI bleeds. Their soft, flexible design allows for precise placement and controlled delivery using the AZUR Detachment System.
ASSOCIATED PRODUCTS
REFERENCES
RX ONLY. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.
- Pelage JP. Angiographic and Pathologic Comparison of HydroCoils vs. Fibered Coils Mechanisms of Occlusion and Mid-Term Recanalization in an Animal Model. GEST. 2012 (animal study)
- Plenk H, Killer M, Richling B. Pathophysiologic considerations on HydroCoil- and platinum coil-occluded retrieved human cerebral aneurysms. Presented at ASITN MicroVention Symposium. 2005 (in-vivo study)
- Data on File. Terumo Corporation. IMS Data.
*Instructions For Use PD110323 Rev. C Revised 2014-04 and AZUR CX 35 IFU PD111138 Rev. A Revised 2015-06